Ready to Deploy On-Premises Redaction Software for PHI Face Redaction?
by Hassaan Mazhar, Last updated: January 2, 2026, ref:
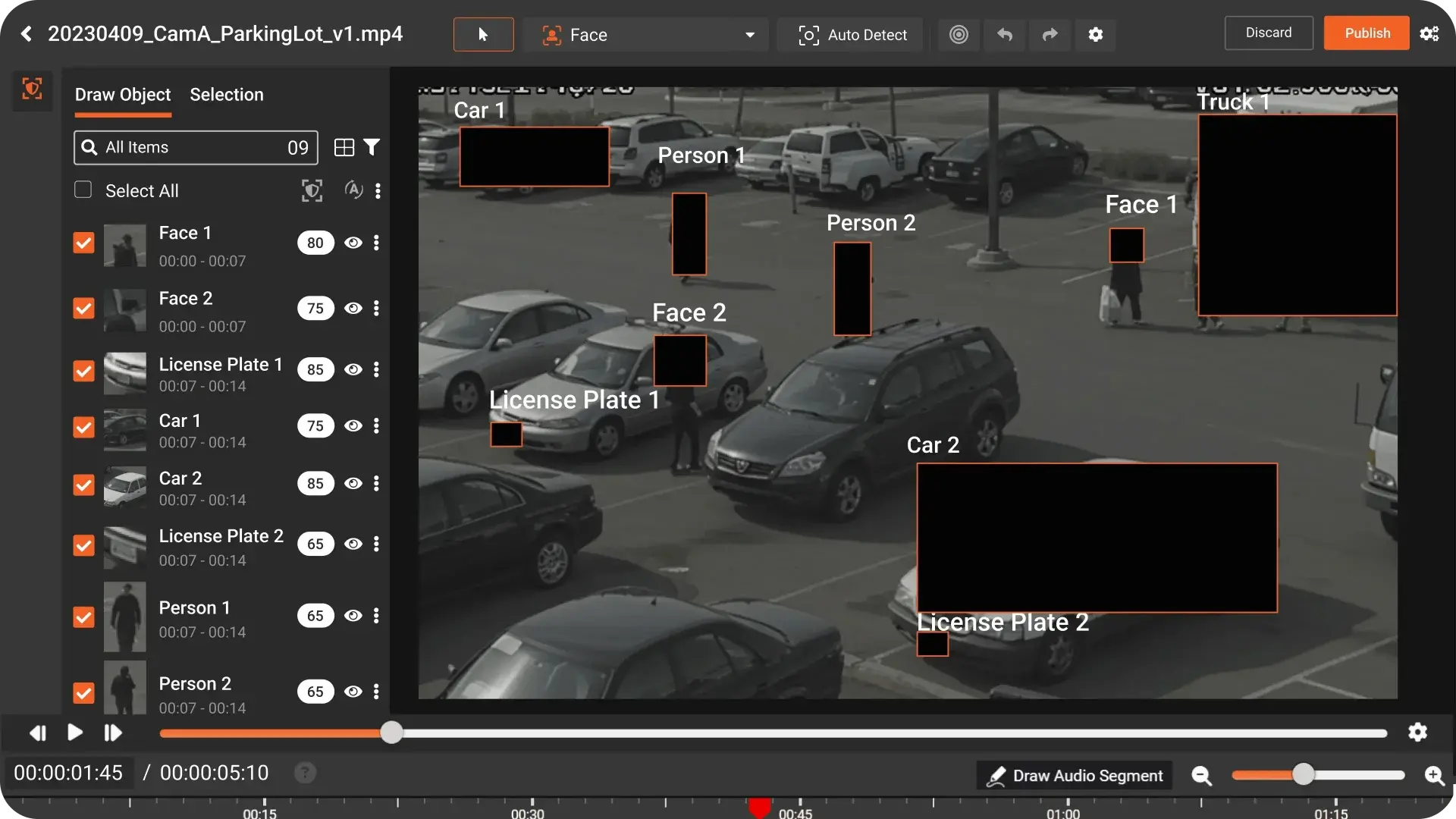
Your pilot proved it works. Faces are blurred, PHI is protected, stakeholders are nodding. But your inbox still fills with last-minute redaction requests, and your team is quietly panicking about how this will scale the moment you flip to production.
This isn’t about whether AI can find faces. It’s about whether your hospital can reliably operationalize on-premises redaction software across hundreds of hours of video and clinical workflows without breaking HIPAA, burning out staff, or stalling other strategic projects.
You Don’t Have a Technology Problem. You Have a Production Problem.
By the time you’re reading this, you’ve already evaluated multiple tools, run proofs of concept, maybe piloted a cloud solution, and learned the basics of on-premises PHI face redaction. The question now is not: “Does it work?”
The question is: “Can we trust this in production, at scale, under real pressure?”
That’s where most hospital teams get stuck:
- Security wants airtight secure on-premises video redaction with no PHI leaving the environment.
- Compliance needs auditability and clear HIPAA-safe workflows.
- Operations demands predictable SLAs and measurable throughput.
- Finance wants cost clarity beyond pilot pricing and per-project scraping.
Everyone is bought in conceptually. No one wants to own the decision to actually go live.
The Real Business Pain: PHI Everywhere, Redaction Nowhere
Let’s be direct: the pain isn’t theoretical risk. It’s the daily grind your teams already feel.
PHI is embedded in everything now:
- ED and ICU CCTV footage used for quality reviews and investigations
- Bodycam and hallway footage requested in litigation and incident response
- Training videos recorded in real clinical environments
- Patient interactions captured in telehealth and virtual care platforms
- Public records and FOIA face redaction demands increasing in volume and complexity
Without production-ready on-premises redaction software, this shows up as:
- Backlogged requests: Legal, compliance, and risk teams waiting weeks because manual redaction is slow and error-prone.
- Shadow processes: Departments using consumer tools or ad hoc vendors – with unclear HIPAA posture.
- Inconsistent quality: Different people using different tools, with no standard for accuracy or retention.
- Hidden labor costs: Senior clinicians or risk managers spending hours in video timelines instead of doing their actual jobs.
The longer you delay a production-grade on-premises video redaction deployment, the more these problems compound – in regulatory risk, human capital, and lost time-to-response.
Why Pilots Feel Great… and Production Feels Terrifying
Pilot environments are controlled. Production is not.
In the pilot, you likely had:
- Curated, short video clips
- One or two dedicated users
- Minimal integration with real hospital systems
- No real SLA pressure from legal or regulators
In production, you’re facing:
- High-volume ingestion: Terabytes of historical CCTV and clinical recordings.
- Mixed quality content: Low-light footage, occluded faces, masks, pediatric patients.
- Multiple user groups: Legal, compliance, risk, security, and clinical education – all with different needs.
- Hard deadlines: Regulatory, litigation, and FOIA face redaction timeframes that don’t care about your IT constraints.
This is where you feel the gap between a cool demo and a true enterprise redaction deployment.
The underlying anxiety: “If we commit to this, will it actually hold up under the weight of our real workflows?”
Anchor the Strategy: What “Good” Looks Like in Production
Before you finalize procurement, you need a clear definition of success for on-premises redaction software. Not high-level benefits – measurable KPIs that you can track and report on.
For most hospitals, that looks like:
- Turnaround time: 50–80% reduction in average time to deliver redacted video for PHI- or FOIA-related requests.
- Throughput: Ability to process X hours of video per day per node (e.g., 50–100+ hours/day on standard GPU hardware).
- Accuracy: Target detection/occlusion rate for faces and other identifiers (e.g., >95% for frontal faces, strong performance for partially obscured faces).
- Adoption: Number of trained users across legal, risk, and compliance; percentage of requests handled via standard workflow inside your on-premises PHI face redaction platform.
- Compliance: Clear, auditable trails for who accessed what, when, and what was redacted.
- Cost per hour processed: All-in operational cost benchmarked against manual or outsourced redaction.
Without these benchmarks, every conversation about scaling on-premises video redaction becomes subjective and political. With them, you can make clean, data-backed decisions about capacity, headcount, and infrastructure.
Choosing the Right Deployment Model: On-Premises, Air-Gapped, or Hybrid
By this stage, you’ve likely landed on on-premises redaction software as your core requirement. But there are still nuances in how you deploy it.
1. Pure On-Premises (Standard Data Center)
Ideal when:
- You have matured virtualized infrastructure or HCI.
- Your PHI policies allow internal network access within a secure segment.
- You want to keep GPU and storage management aligned with existing IT ops.
Key considerations:
- Allocate dedicated GPU resources for AI-driven healthcare video redaction software workloads.
- Segment the network to isolate redaction processing from general traffic.
- Integrate with internal identity providers for single sign-on and least-privilege access.
2. Air-Gapped Redaction Software Deployment
In high-sensitivity environments (e.g., forensics, internal investigations, certain specialty units), air-gapped redaction software is non-negotiable.
That means:
- No internet connectivity required for processing or management.
- Controlled media import/export via secure transfer protocols or offline media.
- Local update mechanisms with signed, pre-approved packages.
This model still benefits from all the features of on-premises redaction software – just hardened for the strictest security posture.
3. Hybrid Deployment
Hybrid makes sense when:
- Some non-PHI workloads can safely leverage cloud for burst capacity.
- You have historical backlogs that exceed on-prem capacity in the short term.
- You want cloud analytics or search, but keep PHI redaction firmly on-prem.
Done right, hybrid lets you keep HIPAA compliant face redaction strictly on-premises while still using cloud where regulations and risk tolerance allow.
Operationalizing On-Premises Redaction Software: From Project to Program
Production success is less about features, more about how you structure the program. You’re not buying a tool; you’re standing up a critical capability.
Define Ownership and Governance
- Business owner: Usually Privacy, Risk, or Compliance – accountable for policy and outcomes.
- Technical owner: IT or clinical engineering – accountable for availability, performance, and integrations.
- Steering group: Legal, Security, and key operational stakeholders – meets regularly to refine workflows and rules of engagement.
Standardize Intake and Workflow
To avoid chaos, define how requests enter the system and how they move:
- Single intake channel for redaction requests (ticketing, service portal, etc.).
- Standard metadata: case ID, requester, legal basis, deadline, PHI sensitivity.
- Clear triage criteria for priority and routing.
- Documented SOPs for standard vs. escalated cases.
Your on-premises redaction software platform should support this with role-based access, configurable workflows, and detailed reporting.
Train for Precision, Not Just Button Clicking
AI accelerates redaction, but humans stay in the loop. Train users to:
- Review and correct AI-generated face detection frames.
- Handle edge cases: partial faces, reflections, badges, screens.
- Use annotation tools effectively for non-face PHI.
- Apply consistent masking policies aligned with your HIPAA interpretation.
Security, Compliance, and Auditability: Proving You’re HIPAA-Ready
Security and compliance teams are often the last barrier between pilot and production. Your job is to give them specifics, not comfort language.
A HIPAA-ready on-premises redaction software implementation should offer:
- End-to-end encryption (in transit and at rest) with modern protocols.
- Role-based access control and granular permissions.
- Detailed audit logs for every action: upload, view, edit, export, delete.
- Configurable retention policies aligned with your legal and records strategy.
- Support for secure on-premises video redaction in segmented networks and VPCs.
For HIPAA compliant face redaction, it’s not enough that faces are blurred. You need to prove:
- Who accessed original, unredacted footage.
- Who approved the final redacted output.
- How and where files moved during processing.
Strong healthcare video redaction software should give you this audit trail out of the box, not as a custom project.
Scaling for FOIA, Risk, and Backlogs: Performance You Can Actually Plan Around
Once you deploy on-premises redaction software, demand almost always grows. Legal realizes this is finally doable. Quality and risk find new use cases. Suddenly your pilot throughput projections are obsolete.
To avoid performance surprises, focus on:
Capacity Planning
- Baseline how many hours of video you must support per month for PHI and FOIA face redaction.
- Map GPU, CPU, and storage requirements per processing node.
- Plan for 2–3x headroom vs. current demand to absorb surges and backlogs.
Elasticity and Parallelism
Your platform should handle:
- Parallel processing of multiple long videos (not just short clips).
- Queue management and prioritization for urgent cases.
- Automated workload distribution across nodes.
In practice, mature healthcare video redaction software will deliver predictable performance benchmarks (e.g., average processing time per hour of HD footage, with and without GPU acceleration). Use this to build realistic SLAs.
Pricing Drivers That Actually Matter (and How to Avoid Surprises)
Late-stage buyers don’t want vague pricing tiers. You want to understand what really drives cost in on-premises redaction software so you can budget with confidence.
Key pricing drivers typically include:
- Volume: Projected number of hours processed per month or year.
- Features: Face-only vs. full-object redaction, audio redaction, annotation, workflow automation.
- Deployment complexity: Standard on-prem vs. hardened air-gapped redaction software setup, HA requirements, and integrations.
- User scale: Number of named or concurrent users across departments.
- Support level: Standard vs. premium support, 24/7 coverage, and dedicated success resources.
Be wary of models that look cheap in pilot but spike with production usage. A transparent enterprise redaction deployment model should align pricing with your real-world risk exposure and redaction needs, not trap you with unpredictable overages.
Implementation Phases: A Realistic Path to Go-Live in 60–90 Days
By now, you’re not looking for theoretical guidance. You want a clear, executable path from decision to production.
A practical implementation roadmap for on-premises redaction software usually looks like this:
Phase 1: Planning & Design (2–3 weeks)
- Validate deployment model (standard on-prem, air-gapped, or hybrid).
- Confirm infrastructure requirements (compute, GPU, storage, network).
- Define initial scope: departments, use cases, and priority workflows.
- Finalize KPIs and reporting expectations.
Phase 2: Install & Integrate (2–4 weeks)
- Deploy the platform in your chosen environment.
- Integrate identity management and access control.
- Configure security baselines, encryption, and audit settings.
- Test ingest and export paths for typical file types and locations.
Phase 3: Pilot-in-Production (2–4 weeks)
- Run real cases end-to-end under monitored conditions.
- Refine workflows and templates for on-premises PHI face redaction.
- Collect baseline metrics for turnaround time and accuracy.
- Adjust capacity and SOPs based on observed patterns.
Phase 4: Full Rollout & Optimization (ongoing)
- Onboard additional departments and user groups.
- Formalize SLAs and escalation paths for urgent requests.
- Review performance and usage quarterly; tune infrastructure as needed.
- Extend into adjacent use cases (training, research, external sharing).
The result: a production-grade, HIPAA compliant face redaction capability that your teams actually trust and use – not another abandoned pilot.
Why VIDIZMO REDACTOR Is the Natural Next Step for Production
At this stage, you don’t need another vendor pitch. You need a platform that behaves the way your hospital operates: controlled, auditable, and resilient under pressure.
VIDIZMO REDACTOR is built for exactly this moment – when you’re ready to move from experimentation to institutional capability with on-premises redaction software. It offers:
- Mature on-premises video redaction and hybrid deployment models, including fully supported air-gapped redaction software configurations.
- High-accuracy, AI-assisted healthcare video redaction software optimized for PHI and clinical environments.
- Enterprise-grade security, auditability, and governance aligned with HIPAA expectations.
- Scalability to handle both live FOIA and legal requests and deep historical backlogs.
- Transparent pricing tied to your real deployment, feature, and volume needs.
If your organization is past evaluation and pilot, and you’re looking for a confident path to deploy on-premises redaction software in production, this is the point where VIDIZMO REDACTOR moves from “interesting option” to “operational requirement.”
People Also Ask:
1. How quickly can we realistically go live with on-premises redaction software?
Most hospitals can move from contract signature to first production use in 60–90 days, assuming infrastructure is available and stakeholders are aligned. A focused implementation plan – with clear owners in IT, compliance, and legal – is more important than the tool itself in hitting that timeline.
2. What infrastructure do we need to support PHI face redaction on-premises?
At a minimum, you’ll need server capacity with dedicated GPU resources (for efficient AI processing), sufficient storage for raw and processed video, and secure network segmentation. Exact specs depend on your target throughput (hours/month) and concurrency needs, which should be sized during planning.
3. How do we prove HIPAA compliance with on-premises PHI face redaction?
Compliance comes from your total implementation: technical controls (encryption, RBAC, audit logs), documented policies (who can do what, under which legal basis), and consistent workflows. Your on-premises redaction software must support detailed auditing, access control, and retention policies that you can map to your HIPAA risk analysis.
4. Can we support both FOIA and internal PHI workflows on the same platform?
Yes, provided the platform supports multi-tenant or multi-workspace configurations with strong role-based access controls. Many hospitals run FOIA, litigation support, and internal quality-improvement redaction workflows side by side, with strict separation of users and cases.
5. How do we handle video from older systems with low resolution or odd formats?
Production-ready healthcare video redaction software should ingest a wide range of legacy formats and resolutions. However, low-quality sources can affect detection performance. Your implementation should include testing against representative legacy footage and SOPs for manual review in difficult cases.
6. What happens if our volume suddenly spikes (e.g., a major incident or litigation)?
This is where capacity planning and scalable architecture matter. With properly sized on-prem nodes – and, optionally, a hybrid burst model for non-PHI content – you can absorb spikes by running more jobs in parallel and prioritizing urgent cases using workflow queues.
7. How do we measure whether our redaction deployment is actually successful?
Track a small set of KPIs: average turnaround time per request, hours of video processed per month, detection accuracy/exception rate, number of users actively submitting and processing cases, and cost per processed hour vs. your old manual or outsourced approach. Review quarterly and adjust capacity and workflows accordingly.
8. What are the typical hidden costs we should watch for?
Hidden costs often show up in infrastructure (underestimated GPU/storage needs), ad hoc custom integrations that weren’t scoped up front, or usage-based pricing that penalizes success. Ensure your vendor is explicit about pricing drivers, support tiers, and any components not included in your initial quote.
9. How do we avoid adoption problems after go-live?
Adoption issues usually come from unclear ownership and fragmented workflows. Designate a business owner, define a single intake process for redaction requests, train a core group of power users in each department, and report back early wins (improved turnaround time, reduced backlog) to reinforce behavior.
Jump to
You May Also Like
These Related Stories

Bulk Redaction Software: Complete Guide for High-Volume Redaction

Addressing PII Redaction Challenges with An Advanced Redaction Software



No Comments Yet
Let us know what you think